Certificate of analysis
How to Interpret
There is an overwhelming amount of information on a Certificate of Analysis (COA). So in order to better help you make an informed decision about the products you use to improve your health, we have provided a sample COA and broke it down piece by piece. Refer to the images and the coordinating numbers with explanations below.


1. Producer Information
-
The name, address, and contact information of the producer of the product being tested by the lab.
2. Sample Identification number
-
This number is used by the laboratory to track the sample. It includes the strain name tested, batch size tested, date sample was received by lab technician, and date report was created.
3. Batch Information
-
This includes the date harvested, strain name, and what type of product is being tested.
4. QR Code
-
A QR code is provided so that faster accesses to the official COA from the lab is obtainable by the consumer using a smart phone and app.
5. △9 THC Total
-
This block provides the △9 THC amount as regulated by Maryland Department of Agriculture. A result of ND= non-detectable. Typically this is a percentage on a dry weight basis, post-decarboxylation.
6. CBD Total
-
This block provides the CBD total of the sample. Typically this is a percentage on a dry weight basis, post-decarboxylation.
7. Cannabinoid Content
-
This is also known as the potency of the product. This is where you find information on what cannabinoids have been detected and in what quantity. The information is especially important for people to know so that they can determine the exact dose that they are taking in the event it needs to be adjusted. It is also important to know if the consumer does not want a specific cannabinoid, such as a high level of THC, in their product.
-
For flower products, dosage is typically presented in a percent by weight, e.g. 10%CBD.For non-flower products, such as topicals, dosage information is typically presented in mg/g or mg/mL. Patitents may then use this information to determine how much is in a dose that they are taking.
8. Notes
-
The first line of notes is an example of the equations that can be used to calculate the potential Total THC and Total CBD (i.e. Total THC = (THCa x 0.877)+△9 THC; Total CBD = (CBDa x 0.877)+CBD)
-
This shows the testing methods for the COA and also lists the LOD and LOQ. LOD is the Limit of Detection, which is the smallest amount that the instrument can accurately identify. LOQ is the Limit of Quantitation, which is the smallest amount that the instrument can accurately quantify.
9. Testing Laboratory Information
-
This is where the third-party testing laboratories information is listed. The physical address, phone number, web address, and license number are found here.
10. Signature Marks
-
All tests should be reviewed by the laboratory prior to release. This test report has been reviewed and approved by the Lab Director.
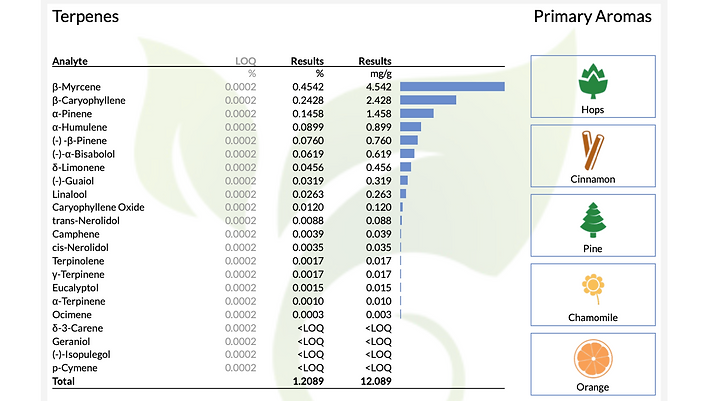
11. Terpene Content
-
Terpenes impart flavors and scents to hemp and contribute to the entourage effect.
12. Aromas
-
This area provides a description of the primary aromas that were tested in the given sample and are listed from top to bottom as most abundant to least.
13. Total Terpenes
-
The total terpenes provides a percentage based on a dry weight basis of the sample tested.
14. Limit of Quantitation (LOQ)
-
The LOQ is the smallest amount that the instrument can accurately quantify. In this case the smallest amount that can be accurately quantified is 20 PPM or as a percent is 0.002%
